
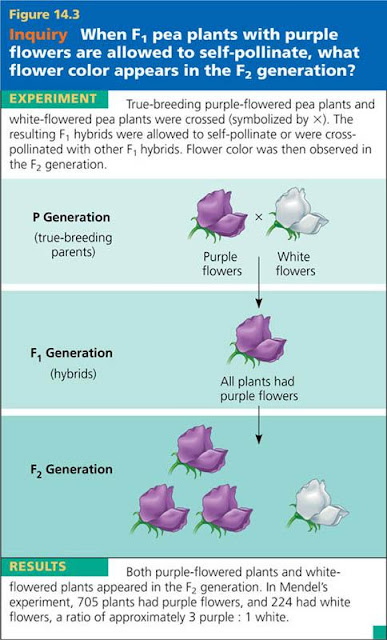
Alleles, alternative versions of a gene. A somatic cell has two copies of each chromosome (forming a homologous pair) and thus two alleles of each gene, which may be identical or different. This figure depicts an F1 pea hybrid with an allele for purple flowers, inherited from one parent, and an allele for white flowers, inherited from the other parent.
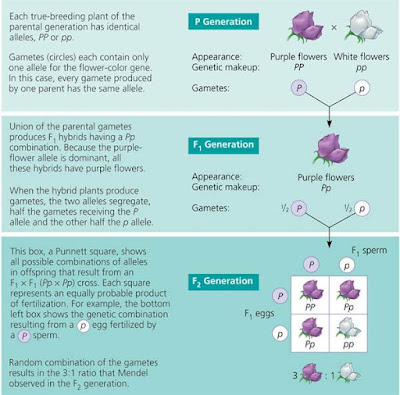
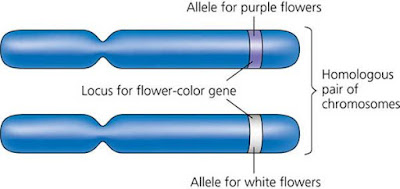
Mendel’s law of segregation. This diagram shows the genetic makeup of the generations in Figure 14.3. It illustrates Mendel’s model for inheritance of the alleles of a single gene. Each plant has two alleles for the gene controlling flower colour, one allele inherited from each parent. To construct a Punnett square, list all the possible female gametes along one side of the square and all the possible male gametes along an adjacent side. The boxes represent the offspring resulting from all the possible unions of male and female gametes.

Phenotype versus genotype. Grouping F2 offspring from a cross for flower color according to phenotype results in the typical 3:1 phenotypic ratio. In terms of genotype, however, there are actually two categories of purple–flowered plants, PP (homozygous) and Pp (heterozygous), giving a 1:2:1 genotypic ratio.


Pedigree Analysis
Unable to manipulate the mating patterns of people, geneticists must analyze the results of matings that have already occurred. They do so by collecting information about a family’s history for a particular trait and assembling this information into a family tree describing the interrelationships of parents and children across the generations—the family pedigree shows a three–generation pedigree that traces the occurrence of a pointed contour of the hairline on the forehead. This trait, called a widow’s peak, is due to a dominant allele, W. Because the widow’s–peak allele is dominant, all individuals who lack a widow’s peak must be homozygous recessive (ww ). The two grandparents with widow’s peaks must have the Ww genotype, since some of their offspring are homozygous recessive. The offspring in the second generation who do have widow’s peaks must also be heterozygous, because they are the products of Ww × ww matings. The third generation in this pedigree consists of two sisters. The one who has a widow’s peak could be either homozygous (WW ) or heterozygous (Ww ), given what we know about the genotypes of her parents (both Ww).
Cystic Fibrosis
The most common lethal genetic disease in the United States is cystic fibrosis, which strikes one out of every 2,500 people of European descent but is much rarer in other groups. Among people of European descent, one out of 25 (4%) is a carrier of the cystic fibrosis allele. The normal allele for this gene codes for a membrane protein that functions in chloride ion transport between certain cells and the extracellular fluid. These chloride transport channels are defective or absent in the plasma membranes of children who inherit two recessive alleles for cystic fibrosis. The result is an abnormally high concentration of extracellular chloride, which causes the mucus that coats certain cells to become thicker and stickier than normal. The mucus builds up in the pancreas, lungs, digestive tract, and other organs, leading to multiple (pleiotropic) effects, including poor absorption of nutrients from the intestines, chronic bronchitis, foul stools, and recurrent bacterial infections. Recent research indicates that the extracellular chloride also contributes to infection by disabling a natural antibiotic made by some body cells. When immune cells come to the rescue, their remains add to the mucus, creating a vicious cycle.
If untreated, most children with cystic fibrosis die before their fifth birthday. Gentle pounding on the chest to clear mucus from clogged airways, daily doses of antibiotics to prevent infection, and other preventive treatments can prolong life. In the United States, more than half of the people with cystic fibrosis now survive into their late 20s or even 30s and beyond.
Sickle–Cell Disease
The most common inherited disorder among people of African descent is sickle–cell disease, which affects one out of 400 African–Americans. Sickle–cell disease is caused by the substitution of a single amino acid in the hemoglobin protein of red blood cells. When the oxygen content of an affected individual’s blood is low (at high altitudes or under physical stress, for instance), the sickle–cell hemoglobin molecules aggregate into long rods that deform the red cells into a sickle shape (see Figure 5.21). Sickled cells may clump and clog small blood vessels, often leading to other symptoms throughout the body, including physical weakness, pain, organ damage, and even paralysis. The multiple effects of a double dose of the sickle–cell allele are another example of pleiotropy. Regular blood transfusions can ward off brain damage in children with sickle–cell disease, and new drugs can help prevent or treat other problems, but there is no cure.
Although two sickle–cell alleles are necessary for an individual to manifest full–blown sickle–cell disease, the presence of one sickle–cell allele can affect the phenotype. Thus, at the organismal level, the normal allele is incompletely dominant to the sickle–cell allele. Heterozygotes, said to have sickle–cell trait, are usually healthy, but they may suffer some sickle–cell symptoms during prolonged periods of reduced blood oxygen. At the molecular level, the two alleles are codominant; both normal and abnormal (sickle–cell) hemoglobins are made in heterozygotes.
About one out of ten African–Americans has sickle–cell trait, an unusually high frequency of heterozygotes for an allele with severe detrimental effects in homozygotes. One explanation for this is that a single copy of the sickle–cell allele reduces the frequency and severity of malaria attacks, especially among young children. The malaria parasite spends part of its life cycle in red blood cells (see Figure 28.11), and the presence of even heterozygous amounts of sickle–cell hemoglobin results in lower parasite densities and hence reduced malaria symptoms. Thus, in tropical Africa where infection with the malaria parasite is common, the sickle–cell allele is both boon and bane. The relatively high frequency of African–Americans with sickle–cell trait is a vestige of their African roots.
Mating of Close Relatives
When a disease–causing recessive allele is rare, it is relatively unlikely that two carriers of the same harmful allele will meet and mate. However, if the man and woman are close relatives (for example, siblings or first cousins), the probability of passing on recessive traits increases greatly. These are called consanguineous (“same blood”) matings, and they are indicated in pedigrees by double lines. Because people with recent common ancestors are more likely to carry the same recessive alleles than are unrelated people, it is more likely that a mating of close relatives will produce offspring homozygous for recessive traits—including harmful ones. Such effects can be observed in many types of domesticated and zoo animals that have become inbred.
There is debate among geneticists about the extent to which human consanguinity increases the risk of inherited diseases. Many deleterious alleles have such severe effects that a homozygous embryo spontaneously aborts long before birth. Still, most societies and cultures have laws or taboos forbidding marriages between close relatives. These rules may have evolved out of empirical observation that in most populations, stillbirths and birth defects are more common when parents are closely related. Social and economic factors have also influenced the development of customs and laws against consanguineous marriages.
Dominantly Inherited Disorders
Although many harmful alleles are recessive, a number of human disorders are due to dominant alleles. One example is achondroplasia, a form of dwarfism with a prevalence of one among every 25,000 people. Heterozygous individuals have the dwarf phenotype
Therefore, all people who are not achondroplastic dwarfs—99.99% of the population—are homozygous for the recessive allele. Like the presence of extra fingers or toes mentioned earlier, achondroplasia is a trait for which the recessive allele is much more prevalent than the corresponding dominant allele.
Dominant alleles that cause a lethal disease are much less common than recessive alleles that do so. All such lethal alleles arise by mutations (changes to the DNA) in a sperm or egg; presumably, such mutations occur equally often whether the mutant allele is dominant or recessive. However, if a lethal dominant allele causes the death of offspring before they mature and can reproduce, the allele will not be passed on to future generations. In contrast, a lethal recessive allele can be perpetuated from generation to generation by heterozygous carriers who have normal phenotypes. These carriers can reproduce and pass on the recessive allele. Only homozygous recessive offspring will have the lethal disease.
A lethal dominant allele can escape elimination if it causes death only at a relatively advanced age. By the time the symptoms become evident, the individual may have already transmitted the lethal allele to his or her children. For example, Huntington’s disease , a degenerative disease of the nervous system, is caused by a lethal dominant allele that has no obvious phenotypic effect until the individual is about 35 to 45 years old. Once the deterioration of the nervous system begins, it is irreversible and inevitably fatal. Any child born to a parent who has the allele for Huntington’s disease has a 50% chance of inheriting the allele and the disorder. (The mating can be symbolized as Aa × aa, with A being the dominant allele that causes Huntington’s disease.) In the United States, this devastating disease afflicts about one in 10,000 people.
Until relatively recently, the onset of symptoms was the only way to know if a person had inherited the Huntington’s allele. This is no longer the case. By analyzing DNA samples from a large family with a high incidence of the disorder, geneticists tracked the Huntington’s allele to a locus near the tip of chromosome 4.

Meiotic nondisjunction. Gametes with an abnormal chromosome number can arise by nondisjunction in either meiosis I or meiosis II.Alterations of chromosome structure. Vertical arrows indicate breakage points. Dark purple highlights the chromosomal parts affected by the rearrangements.
Down syndrome.The child exhibits the facial features characteristic of Down syndrome. The karyotype shows trisomy 21, the most common cause of this disorder.




1 comments:
I liked your input is very nice
Please could you send me the article where you got this information, and graphics please will_2601@hotmail.com
Post a Comment