he plasma membrane is the edge of life, the boundary that separates the living cell from its nonliving surroundings. A remarkable film only about 8 nm thick—it would take over 8,000 to equal the thickness of this page—the plasma membrane controls traffic into and out of the cell it surrounds. Like all biological membranes, the plasma membrane exhibits selective permeability; that is, it allows some substances to cross it more easily than others. One of the earliest episodes in the evolution of life may have been the formation of a membrane that enclosed a solution different from the surrounding solution while still permitting the uptake of nutrients and elimination of waste products. This ability of the cell to discriminate in its chemical exchanges with its environment is fundamental to life, and it is the plasma membrane and its component molecules that make this selectivity possible.
Phospholipid bilayer cross section
More than 50 kinds of proteins have been found so far in the plasma membrane of red blood cells, for example. Phospholipids form the main fabric of the membrane, but proteins determine most of the membrane’s specific functions. Different types of cells contain different sets of membrane proteins, and the various membranes within a cell each have a unique collection of proteins.
Synthesis of membrane components and their orientation on the resulting membrane. The plasma membrane has distinct cytoplasmic and extracellular sides, or faces, with the extracellular face arising from the inside face of ER, Golgi, and vesicle membranes.
Membranes have distinct inside and outside faces. The two lipid layers may differ in specific lipid composition, and each protein has directional orientation in the membrane. When a vesicle fuses with the plasma membrane, the outside layer of the vesicle becomes continuous with the cytoplasmic layer of the plasma membrane. Therefore, molecules that start out on the inside face of the ER end up on the outside face of the plasma membrane.
The process starts with (1) the synthesis of membrane proteins and lipids in the endoplasmic reticulum. Carbohydrates (green) are added to the proteins (purple), making them glycoproteins. The carbohydrate portions may then be modified. (2) Inside the Golgi apparatus, the glycoproteins undergo further carbohydrate modification, and lipids acquire carbohydrates, becoming glycolipids. (3) Transmembrane proteins (purple dumbbells), membrane glycolipids, and secretory proteins (purple spheres) are transported in vesicles to the plasma membrane. (4) There the vesicles fuse with the membrane, releasing secretory proteins from the cell. Vesicle fusion positions the carbohydrates of membrane glycoproteins and glycolipids on the outside of the plasma membrane. Thus, the asymmetrical distribution of proteins, lipids, and their associated carbohydrates in the plasma membrane is determined as the membrane is being built by the ER and Golgi apparatus.
The biological membrane is an exquisite example of a supramolecular structure—many molecules ordered into a higher level of organization—with emergent properties beyond those of the individual molecules. The remainder of this chapter focuses on one of the most important of those properties: the ability to regulate transport across cellular boundaries, a function essential to the cell’s existence. We will see once again that form fits function: The fluid mosaic model helps explain how membranes regulate the cell’s molecular traffic.
A steady traffic of small molecules and ions moves across the plasma membrane in both directions. Consider the chemical exchanges between a muscle cell and the extracellular fluid that bathes it. Sugars, amino acids, and other nutrients enter the cell, and metabolic waste products leave it. The cell takes in oxygen for cellular respiration and expels carbon dioxide. It also regulates its concentrations of inorganic ions, such as Na+, K+, Ca2+, and Cl−, by shuttling them one way or the other across the plasma membrane. Although traffic through the membrane is extensive, cell membranes are selectively permeable, and substances do not cross the barrier indiscriminately. The cell is able to take up many varieties of small molecules and ions and exclude others. Moreover, substances that move through the membrane do so at different rates.
Hydrophobic (nonpolar) molecules, such as hydrocarbons, carbon dioxide, and oxygen, can dissolve in the lipid bilayer of the membrane and cross it with ease, without the aid of membrane proteins. However, the hydrophobic core of the membrane impedes the direct passage of ions and polar molecules, which are hydrophilic, through the membrane. Polar molecules such as glucose and other sugars pass only slowly through a lipid bilayer, and even water, an extremely small polar molecule, does not cross very rapidly. A charged atom or molecule and its surrounding shell of water find the hydrophobic layer of the membrane even more difficult to penetrate. Fortunately, the lipid bilayer is only part of the story of a membrane’s selective permeability. Proteins built into the membrane play key roles in regulating transport.
Cell membranes are permeable to specific ions and a variety of polar molecules. These hydrophilic substances can avoid contact with the lipid bilayer by passing through transport proteins that span the membrane. Some transport proteins, called channel proteins, function by having a hydrophilic channel that certain molecules or atomic ions use as a tunnel through the membrane. For example, the passage of water molecules through the membrane in certain cells is greatly facilitated by channel proteins known as aquaporins . Other transport proteins, called carrier proteins, hold onto their passengers and change shape in a way that shuttles them across the membrane. In both cases, the transport protein is specific for the substance it translocates (moves), allowing only a certain substance (or substances) to cross the membrane. For example, glucose carried in blood and needed by red blood cells for cellular activities enters these cells rapidly through specific transport proteins in the plasma membrane. This “glucose transporter” is so selective as a carrier protein that it even rejects fructose, a structural isomer of glucose.
Thus, the selective permeability of a membrane depends on both the discriminating barrier of the lipid bilayer and the specific transport proteins built into the membrane. But what determines the direction of traffic across a membrane? At a given time, will a particular substance enter or leave the cell? And what mechanisms actually drive molecules across membranes? We will address these questions next as we explore two modes of membrane traffic: passive transport and active transport.
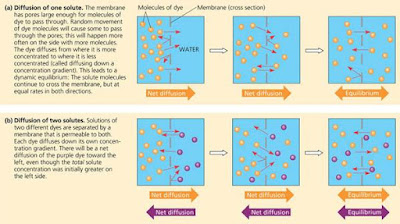
The diffusion of solutes across a membrane. Each of the large arrows under the diagrams shows the net diffusion of the dye molecules of that colour.
Each dye molecule wanders randomly, but there will be a net movement of the dye molecules across the membrane to the side that began as pure water. The dye molecules will continue to spread across the membrane until both solutions have equal concentrations of the dye. Once that point is reached, there will be a dynamic equilibrium, with as many dye molecules crossing the membrane each second in one direction as in the other.
We can now state a simple rule of diffusion: In the absence of other forces, a substance will diffuse from where it is more concentrated to where it is less concentrated. Put another way, any substance will diffuse down its concentration gradient . No work must be done to make this happen; diffusion is a spontaneous process. Note that each substance diffuses down its own concentration gradient, unaffected by the concentration differences of other substances.
Much of the traffic across cell membranes occurs by diffusion. When a substance is more concentrated on one side of a membrane than on the other, there is a tendency for the substance to diffuse across the membrane down its concentration gradient (assuming that the membrane is permeable to that substance). One important example is the uptake of oxygen by a cell performing cellular respiration. Dissolved oxygen diffuses into the cell across the plasma membrane. As long as cellular respiration consumes the O2 as it enters, diffusion into the cell will continue, because the concentration gradient favors movement in that direction.
The diffusion of a substance across a biological membrane is called passive transport because the cell does not have to expend energy to make it happen. The concentration gradient itself represents potential energy and drives diffusion. Remember, however, that membranes are selectively permeable and therefore have different effects on the rates of diffusion of various molecules. In the case of water, aquaporins allow water to diffuse very rapidly across the membranes of certain cells. The movement of water across the plasma membrane has important consequences for cells.
Osmosis. Two sugar solutions of different concentrations are separated by a selectively permeable membrane, which the solvent (water) can pass through but the solute (sugar) cannot. Water molecules move randomly and may cross through the pores in either direction, but overall, water diffuses from the solution with less concentrated solute to that with more concentrated solute. This transport of water, or osmosis, eventually equalizes the sugar concentrations on both sides of the membrane.
Pores in this synthetic membrane are too small for sugar molecules to pass through but large enough for water molecules. How does this affect the water concentration?
It seems logical that the solution with the higher concentration of solute would have the lower concentration of water and that water would diffuse into it from the other side for that reason. However, for a dilute solution like most biological fluids, solutes do not affect the water concentration significantly. Instead, tight clustering of water molecules around the hydrophilic solute molecules makes some of the water unavailable to cross the membrane. It is the difference in free water concentration that is imporant. But the effect is the same: Water diffuses across the membrane from the region of lower solute concentration to that of higher solute concentration until the solute concentrations on both sides of the membrane are equal. The diffusion of water across a selectively permeable membrane is called osmosis. The movement of water across cell membranes and the balance of water between the cell and its environment are crucial to organisms. Let’s now apply to living cells what we have learned about osmosis in artificial systems.
When considering the behaviour of a cell in a solution, both solute concentration and membrane permeability must be considered. Both factors are taken into account in the concept of tonicity , the ability of a solution to cause a cell to gain or lose water. The tonicity of a solution depends in part on its concentration of solutes that cannot cross the membrane (nonpenetrating solutes), relative to that in the cell itself. If there are more nonpenetrating solutes in the surrounding solution, water will tend to leave the cell, and vice versa.
If a cell without a wall, such as an animal cell, is immersed in an environment that is isotonic to the cell (iso means “same”), there will be no net movement of water across the plasma membrane. Water flows across the membrane, but at the same rate in both directions. In an isotonic environment, the volume of an animal cell is stable
The water balance of living cells. How living cells react to changes in the solute concentration of their environment depends on whether or not they have cell walls. (a) Animal cells such as this red blood cell do not have cell walls. (b) Plant cells do. (Arrows indicate net water movement since the cells were first placed in these solutions.)
Now let’s transfer the cell to a solution that is hypertonic to the cell (hyper means “more,” in this case more nonpenetrating solutes). The cell will lose water to its environment, shrivel, and probably die. This is one reason why an increase in the salinity (saltiness) of a lake can kill the animals there—if the lake water becomes hypertonic to the animals’ cells, the cells might shrivel and die. However, taking up too much water can be just as hazardous to an animal cell as losing water. If we place the cell in a solution that is hypotonic to the cell (hypo means “less”), water will enter the cell faster than it leaves, and the cell will swell and lyse (burst) like an overfilled water balloon.
A cell without rigid walls can tolerate neither excessive uptake nor excessive loss of water. This problem of water balance is automatically solved if such a cell lives in isotonic surroundings. Seawater is isotonic to many marine invertebrates. The cells of most terrestrial (land–dwelling) animals are bathed in an extracellular fluid that is isotonic to the cells. Animals and other organisms without rigid cell walls living in hypertonic or hypotonic environments must have special adaptations for osmoregulation , the control of water balance. For example, the protist Paramecium lives in pond water, which is hypotonic to the cell. Paramecium has a plasma membrane that is much less permeable to water than the membranes of most other cells, but this only slows the uptake of water, which continually enters the cell. Paramecium doesn’t burst because it is also equipped with a contractile vacuole, an organelle that functions as a bilge pump to force water out of the cell as fast as it enters by osmosis.
The contractile vacuole of Paramecium: an evolutionary adaptation for osmoregulation. The contractile vacuole of this freshwater protist offsets osmosis by bailing water out of the cell.
The cells of plants, prokaryotes, fungi, and some protists have walls. When such a cell is immersed in a hypotonic solution—bathed in rainwater, for example—the wall helps maintain the cell’s water balance. Consider a plant cell. Like an animal cell, the plant cell swells as water enters by osmosis. However, the elastic wall will expand only so much before it exerts a back pressure on the cell that opposes further water uptake. At this point, the cell is turgid (very firm), which is the healthy state for most plant cells. Plants that are not woody, such as most houseplants, depend for mechanical support on cells kept turgid by a surrounding hypotonic solution. If a plant’s cells and their surroundings are isotonic, there is no net tendency for water to enter, and the cells become flaccid (limp).
However, a wall is of no advantage if the cell is immersed in a hypertonic environment. In this case, a plant cell, like an animal cell, will lose water to its surroundings and shrink. As the plant cell shrivels, its plasma membrane pulls away from the wall. This phenomenon, called plasmolysis, causes the plant to wilt and can be lethal. The walled cells of bacteria and fungi also plasmolyze in hypertonic environments.
Let’s look more closely at how water and certain hydrophilic solutes cross a membrane. As mentioned earlier, many polar molecules and ions impeded by the lipid bilayer of the membrane diffuse passively with the help of transport proteins that span the membrane. This phenomenon is called facilitated diffusion. Cell biologists are still trying to learn exactly how various transport proteins facilitate diffusion. Most transport proteins are very specific: They transport only particular substances but not others.
As described earlier, the two types of transport proteins are channel proteins and carrier proteins. Channel proteins simply provide corridors that allow a specific molecule or ion to cross the membrane
Two types of transport proteins that carry out facilitated diffusion. In both cases, the protein transports the solute down its concentration gradient.
The hydrophilic passageways provided by these proteins allow water molecules or small ions to flow very quickly from one side of the membrane to the other. While water molecules are small enough to cross through the phospholipid bilayer, the rate of water movement by this route is relatively slow because of their polarity. Aquaporins, the water channel proteins, facilitate the massive amounts of diffusion that occur in plant cells and in animal cells such as red blood cells. Another group of channels are ion channels , many of which function as gated channels ; a stimulus causes them to open or close. The stimulus may be electrical or chemical; if chemical, the stimulus is a substance other than the one to be transported. For example, stimulation of a nerve cell by certain neurotransmitter molecules opens gated channels that allow sodium ions into the cell.
Carrier proteins seem to undergo a subtle change in shape that somehow translocates the solute–binding site across the membrane. These changes in shape may be triggered by the binding and release of the transported molecule.
In certain inherited diseases, specific transport systems are either defective or missing altogether. An example is cystinuria, a human disease characterized by the absence of a protein that transports cysteine and some other amino acids across the membranes of kidney cells. Kidney cells normally reabsorb these amino acids from the urine and return them to the blood, but an individual afflicted with cystinuria develops painful stones from amino acids that accumulate and crystallise in the kidneys.








0 comments:
Post a Comment